Take the step .... and secure the regulator’s confidence
As European CRO and Head of Compliance for a global insurer, I experienced first-hand the challenge of gaining and keeping the regulator’s confidence. Now working with Decision Focus, I’ve reflected on what were the biggest headaches and looked at what our product can do to help. Here’s one for which I feel we have developed a novel solution: Regulatory Reporting.
Regulatory reporting challenges
As a European firm we made submissions to 12 regulators in all: the FCA and PRA in the UK and 10 more across Europe. We also had a Lloyd’s Managing Agent, and their business timetable alone cites around 250 potential submissions. Then there were submissions to legal and statutory stakeholders, reinsurance pools, and trade bureaus such as the MIB. Whilst some submissions were annual, others were quarterly. Furthermore, most submissions were made separately for each regulated entity within the group. Our annual submission count was comfortably in the 3-figure zone. So, the first challenge is simply keeping track of what you owe to whom, and when. Our ‘solution’ to this problem was the inevitable spreadsheet, but this was high effort, and error prone. On refection, a poor tool for the job.
Many of the submissions we made were complex and data intense, often requiring effort from multiple contributors to prepare. This required co-ordination and collaboration. Our ‘solution’ to this problem was the ubiquitous email. Simply put, the guardians of the spreadsheet drove progress by sending first messages, then warnings, and finally threats. Looking back, it was too haphazard for safety.
The regulators have always held clear expectations about being on time; if you are late, they fine you. But they also expect that submissions are accurate, so they are reliably informed. Our ‘solution’ for this expectation was our best endeavours. They were our level best, but now I think of it, they were not truly systemised in a way that could be readily evidenced. The regulators have shone a brighter light on this recently with substantial fines for firms whose submissions have lacked the necessary quality. There has also been firm messaging to the market generally through their ‘Dear CEO’ missives.
So, my recollection is of three key challenges: clarity and central control of submission requirements; being on time; and ensuring quality.
Here’s a brief overview of the capabilities we have developed within the Decision Focus GRC solution to tackle these testing goals.
An intelligent repository puts you in control
At the heart of our solution is the ability to define reference submissions in a central repository, i.e. the total list of submission types for the entire group. Each reference submission specifies an instruction set for actual submissions. An example might say ‘We must make submission X for these three entities (perhaps syndicates) in the group, quarterly’.
Now comes the intelligence. From these reference submissions Decision Focus will read the instructions and automatically generate an annual business timetable, with all the required, actual submissions placed in time. It’s viewable as an Outlook style calendar.
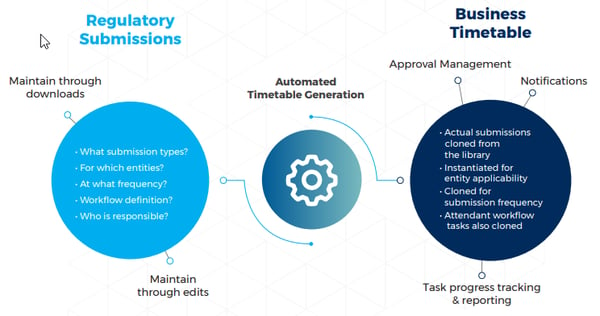
In generating actual submissions in the business timetable from the instructions held in the reference submissions Decision Focus essentially 'clones' the reference submissions as necessary. There are three cases: cloning for entity applicability (we make this submission for three regulated entities) , cloning for frequency (we make this submission quarterly, for a single entity), and a combination of the two (we make this submission for three entities, quarterly).
.png?width=870&name=image%20(1).png)
Nothing stands still, least of all the submission requirements for regulators. With our approach, when the regulator adds, removes, or retimes submissions you simply need to edit the reference submissions. You can then generate the business timetable for next year with a single button push. It's a lot easier to maintain the reference set and let the tool manage the creation of the business timetable, rather than manually maintain the 100s of actual submissions. This brings a massive effort reduction, consistency, and clarity about who needs to do what, and when.
Smart workflow keeps you on time
My experience was effective collaboration was key to being on time. Decision Focus manages this collaboration using workflow techniques. You can create a workflow for each reference submission, defining the preparation tasks required. Each is allocated to the appropriate function e.g. risk, actuarial or finance and the named individuals within each function who will be responsible. Task durations and sequence are also defined.
When you press the button to generate the business timetable, Decision Focus automatically creates the workflow tasks for each actual submission, each with the required start and end dates and allocated to the person responsible. These are visible as a project Gantt chart. In the example above, if the instruction set for ‘submission X’ defines a five-step workflow Decision Focus will create 60 tasks, 5 for each of the 12 actual submissions. In one automated step you have created clear instructions for all the submission preparers, in all locations, to get those 12 submissions delivered on time.
Now it’s a simple matter of getting those tasks done. Decision Focus excels here with comprehensive progress tracking via live dashboards, automated ‘start soon’, ‘due soon’, ‘ready for approval’, ‘approved’ and submitted notifications to the right individuals.
Data quality ensures you are reliable
The solution was staring us in the face. We have simply re-used the data quality capabilities we developed for model validation, applying it to submission preparation. The base datasets on which each submission relies are defined within the tool. The quality criteria each dataset must exhibit are then defined. These defined required degrees of completeness, accuracy, timeliness. Finally, each criterion is mapped to the data quality controls designed and operated to ensure the criteria are met. These sit along with all the other controls, in Decision Focus' integrated control register. Decision Focus supports periodic control assessment and testing to uncover deficiencies and drive remedial action. This is important evidence for the regulator that you have a systemised approach to ensuring the quality of submission contents.
In summary we have done three things: Firstly, our intelligent repository automatically generates the submissions business timetable for all group entities and all the regulatory jurisdictions in which they operate. Secondly, smart workflow techniques co-ordinate all the tasks required to prepare, approve and deliver submissions. Finally, proven data quantity management capabilities ensure the contents of your submissions are reliable.
I think we have added some truly valuable capabilities to the tool to help with the challenges of getting regulatory reporting right, and giving the regulator the confidence they seek. What do you think?




